Chuong Vu's curriculum project focuses on a set of activities that introduce chemistry and polymer science through physical interaction with plastics.
MODELING CHEMICAL STRUCTURE WITH HAND MOLDABLE PLASTIC
Click to view full size:
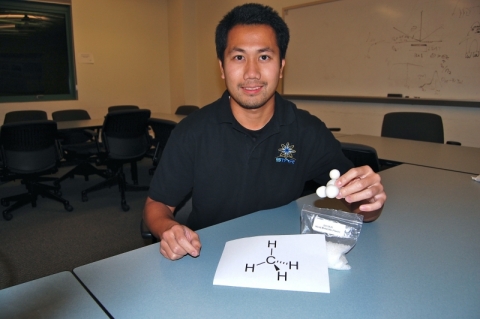
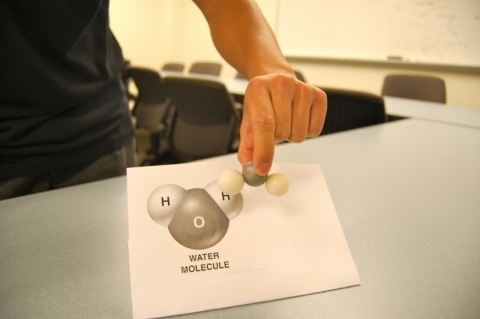
Modeling molecular structure using "ball and stick" kits of snap-together pieces is a time tested approach to introducing chemical structure. This activity differs in that the students create their own pieces from raw material "hand moldable plastic" and small neodymium magnets*. Manipulation of the size of the individual plastic atoms and the inner orientation of the magnets can be used to describe the different bonding patterns and electronic structure of the elemental constituents of the model. For example, for a water molecule the central oxygen atom could be larger than the hydrogen atoms and have two magets in appropriate 109.5 degree geometrical orientation, while the hydrogens can be smaller and contain one magnet.
The melting temperature of this plastic is low enough so that it can be successfully shaped in the classroom (melting temp = 60 deg C, 140 deg F). To melt simply submerge in hot water, we used a beaker on a hot plate. Remove from the water and form by hand, incorporating the magnets or other items. At the end of class the models can be reheated and formed into new shapes by the next class!
Link to Mr. Vu's curriculum activity coming soon...
*please note that there is a safety hazard present in the magnets - if two or more of these rare-earth magnets are swallowed it could result in internal injury.
OOGOO: investigating the properties of silicone/corn starch mixtures
Click to view full size:
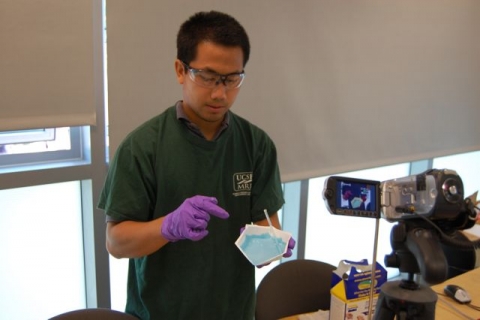
Oogoo is a flexible material of remarkable strength made by mixing 100% pure silicone* with corn starch (the type of silicone commonly used to seal cracks and available at any hardware store). Students investigate the properties of different blends of oogoo. The polymerization reaction of silicone produces acetic acid and results in an unmistakeable vinegar smell. In a well-ventilated room this activity is safe, students should take care not to directly breath in the air directly above mixing container.
Link to Mr. Vu's curriculum activity coming soon...
* for best results use regular/plain silicone like "GE silicone I" not any of the "GE silicone II" or products with additives
SOFT ROBOTS
Click to view full size:
Soft robotics is an exciting field of research pioneered by the Whitesides group at Harvard. The fabrication of the molds and pouring of the elastomers is fairly straighforward, we envision soft robots making their way into high school robotics or other high school science projects. Soft robots usually consist of two types of elastomer, an extremely soft component and a more rigid harder plastic. As air enters the inner chamber of a soft robot it deforms the softer plastic side more than the firm side, resulting in a deflection of the shape.
VIDEO
"making of" videos: (we used a low-cost CNC to cut these molds but its also possible to make these by hand, contact Chuong or Frank for details)
More TBA!