Safinya Group Microscopes

Confocal Microscope
Confocal Microscope
Our laser scanning confocal microscopy (LSCM) system includes an inverted DMIRBE microscope (Leica Inc.) fitted with a motorized lens turret that is also motorized in the z direction, allowing the focus to be adjusted electronically as well as manually. In addition, a piezo z-stage enables very fine movements of the sample in the z direction. The scope is currently equipped with three objectives, 10x dry, 40x oil phase, and 60x water DIC. The scanner allows for simultaneous scanning of four channels. Light sources: halogen lamp (transmission contrast); 50W Hg arc lamp (epi-fluorescence). Laser sources: argon, krypton, and helium-neon lasers (single photon events, i.e., regular LSCM); tunable Tsunami laser (Spectra Physics) with midrange optics, with output wavelengths between 710 nm and 900 nm and maximum intensity at around 810 nm (multiphoton events). More information about the microscope can be found on the MRL Facility webpage.
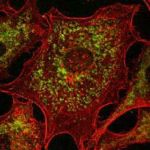
Cells with fluorescently
stained organelles and
actin cytoskeleton

Bone-shaped
Block-Liposomes (DIC)
Inverted Microscope
The inverted microscope Diaphot 300 (Nikon) has been developed into a highly versatile machine. Objectives: 10x dry, 20x dry DIC, 40x dry phase, and 60x oil DIC. Light sources: halogen; 100W Hg arc; 150W Xe arc. Cameras: CCD (Sony); VE1000 (Dage) for high resolution; VE1000SIT (Dage) for fluorescence. Image acquisition can be further improved using the integrator/processor DSP2000 (Dage) and the NIH Image software. Various setups have been developed on the scope, including microinjection.

Inverted Microscope

Upright Microscope
Upright Microscope
The Nikon Microphot-Fx upright microscope can be used in either reflection or transmission mode. As shown in this figure, a microinjector and humidity-control apparatus are often assembled for use with the microchannel project. This camera is fully equipped for DIC and epi-fluorescence and has 10x, 20x, and 40x objectives. A CCD camera routes to a video monitor and either single frame or time-series data can be saved on a super-VHS recorder. Alternatively, a Nikon CoolPix digital camera can collect JPEG images.

Liquid Crystal in
Microchannels
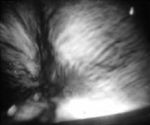
Neurofilament Network
viewed between crossed polarizers
MRL Microscope
The MRL microscope is a general use instrument that is currently set up as a recharge facility. This Nikon Optiphot is equipped with 5x, 10x, 20x and 40x objectives, as well as two light sources allowing for both reflection and transmission microscopy. In addition, this microscope is ideal for crossed-polarized microscopy. The standard camera is a CCD that routes to a video monitor. Images can be saved on super-VHS video or captured with either a black and white or color video printer. On request, a Nikon CoolPix digital camera can be used to collect jpeg images. Sign-up is on a first-come, first-served basis. A description of how to align the microscope can be found here.

MRL Microscope