|
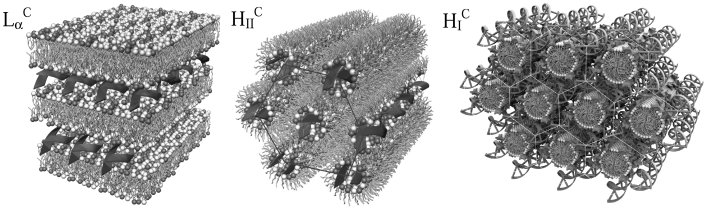
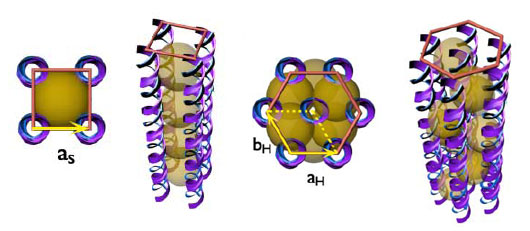
Introduction Recent Reviews Safinya, C. R.; Ewert, K. K.; Leal, C.: Cationic liposome-nucleic acid complexes: liquid crystal phases with applications in gene therapy. Liq. Cryst. 2011, 38, 1715-1723. DOI: 10.1080/02678292.2011.624364. Ewert, K. K.; Zidovska, A.; Ahmad, A.; Bouxsein, N. F.; Evans, H. M.; McAllister, C. S.; Samuel, C. E.; Safinya, C. R.: Cationic Lipid–Nucleic Acid Complexes for Gene Delivery and Silencing: Pathways and Mechanisms for Plasmid DNA and siRNA. Topics Curr. Chem. 2010, 296, 191-226. DOI: 10.1007/128_2010_70. Lipid-DNA Interactions: Structure-Function Studies of Nanomaterials for Gene Delivery: Ewert, K. K.; Samuel, C. E. and Safinya, C. R. in Interaction of DNA with Surfactant and Polymers, pp. 377-404; Eds.: R. Dias, B. Lindman; Blackwell, Boston, MA, 2008. Motivation Our work on nucleic acid (NA, i.e. DNA and RNA) delivery is motivated by the prospects of gene therapy. Gene therapy aims to cure both inherited and acquired diseases by adding, replacing, or correcting genes through the introduction of foreign NAs. In this new approach to medicine, diseases which have a genetic basis can be cured at their root, instead of just mitigating their symptoms. Obvious targets are inherited diseases, but an even more important target are diseases caused by an aquired genetic defect (e.g. cancers). In addition, many other diseases can be targeted with gene therapy, with such prominent targets as HIV/AIDS, Parkinsons and cardiovascular diseases among them. Following the recent breakthrough discoveries in RNA interference (see Nobel Prize in medicine 2007!), gene therapy can now not only add or repair genes, but also switch unwanted genes off. All these prospects depend on a viable, efficient and safe delivery system (or vector) for NAs. A large number of clinical trials in gene therapy are being conducted worldwide, with the majority of them in the treatment of cancer. Some successes have been achieved, but there have also been some serious setbacks. It is generally agreed, however, that the currently available NA vectors leave much to be desired from the clinician's perspective. Vectors for Gene Delivery The future of gene therapy depends on the ability to successfully and efficiently transfer and express exogenous DNA in somatic cells without unwanted side effects. From the time the concept of gene therapy was proposed, genetically altered viruses have been used as vectors. Due to their superior efficiency, especially in vivo (optimized by many thousands of years of evolution), altered viruses still constitute the majority of vectors used in clinical trials. There are, however, mounting concerns regarding the safety of viral vectors. In addition, these vectors trigger an immune response which may prevent repeated administrations, and the capacity of viral vectors is very limited (around 40 kbp) due to their small genome size. Mechanisms of and Barriers to Gene Delivery By looking at the way by which viruses are able to deliver their DNA to a cell, we can establish design criteria for an ideal gene delivery vector. In short, a successful vector or carrier should: This is a very complex task, and the field of CL–DNA complexes probably owes its existence to the serendipitous fact that cationic lipids are able to perform several of these tasks reasonably well, at least in vitro. Future lipid vectors will likely incorporate several components, each designed to optimally perform one of these tasks. Cationic Lipid–DNA Complexes Cationic lipids (CL) are promising vectors for gene delivery to cells and a desirable alternative to viral vectors. Most lipid-based vectors investigated to date consist of mixtures of a cationic with a neutral lipid. Typically, mixed liposomes of the neutral and cationic lipid are formed first and then combined with DNA, which leads to the spontaneous formation of CL-DNA complexes A large number of structurally quite diverse cationic lipids have been synthesized by groups all over the world in efforts to improve the efficiency of CL–DNA complexes. A large amount of work has also been performed in order to understand the formation of cationic lipid–DNA complexes, their structure and their mechanism of action. Importantly, the fact that CL–DNA complexes are self-assembled, supramolecular structures makes it hard to directly correlate lipid chemical structure with the efficiency of DNA delivery. Instead, important properties of an optimized lipid gene delivery vehicle may be mediated by physical-chemical properties of the lipid self-assembly. Structures of Lipid–DNA Complexes and their Effect on Transfection The initial hypothesis on the structure of CL–DNA complexes assumed a simple condensation of liposomes and DNA, resulting in a "spaghetti and meatball" picture. In 1997 and 1998, however, our x-ray diffraction work showed that a major structural rearrangement takes place [1,2], resulting in disruption of the liposomes. Two different structures were observed for CL–DNA complexes containing DOTAP as the cationic lipid, depending on the nature of the neutral lipid and the lipid composition. Figure 1 shows the nanoscale local structure of the lamellar and the inverted hexagonal phases of CL–DNA complexes. While the inverted hexagonal structure requires the neutral lipid DOPE (which has a molecular shape that favors the inverted micelles present in the structure), the lamellar phase is very common and has been observed for the vast majority of the complexes studied so far. Recently, we have discovered a third phase of CL–DNA complexes, the hexagonal structure, which consists of cylindrical lipid micelles embedded in a honeycomb lattice of DNA. This structure (also shown in Figure 1) is only observed with a lipid that has a very large and highly charged headgroup, favoring the formation of micelles (See Lipid Synthesis) [3].
Significant differences in the transfection properties of hexagonal and lamellar complexes have been reported and we have proposed models for their different modes of action [4]. Other Parameters Affecting the Efficiency of CL–DNA Vectors A few compositional parameters of CL–DNA complexes affect their transfection efficiency (TE) strongly. One of these is the lipid/DNA charge ratio (ρchg). For all lipids investigated in our laboratory to date, TE increases with ρchg up to a saturation value; this behavior is independent from the ratio of cationic to neutral lipid in the membrane. The investigated lipids cover headgroup charges from +1 to +16 and varied headgroup structures. The onset of the saturation depends on the cationic lipid. For example, as shown in Fig. 2, ρchg = 3 lies in the saturated regime for DOTAP, while a group of recently synthesized dendritic lipids required at least ρchg = 4.5 [5]. Fig. 2 displays the transfection efficiencies of complexes with 60 mol% cationic lipid for DOTAP (+1), MVLG2 (+4), MVLBG1 (+8) and MVLBG2 (+16) at various values of ρchg.
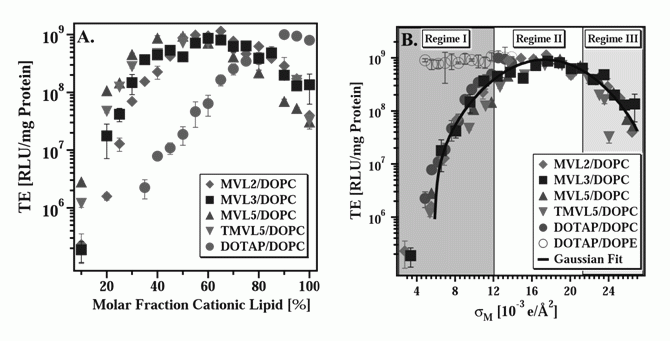
A second key parameter affecting TE of CL–DNA complexes is the membrane charge density (σM), which varies with the ratio of cationic and neutral lipid in the membrane. The membrane charge density provides a lipid-independent measure of "how cationic" a membrane is, since it is defined simply as the cationic charge per unit area. For example, two membranes, each containing the same molar fraction of a cationic lipid, may exhibit very different values of σM if the two cationic lipids carry a different charge (assuming their headgroup areas are the same). At the same time, σM of two membranes containing very different molar fractions of cationic lipid may be similar, if the lipids bear very different charges. To calculate the membrane charge density, one needs to know the effective charge of the cationic lipid in DNA complexation (which can be obtained using an ethidium bromide based assay) and the lipid's headgroup area, which we use as a fitting parameter [6]. The remarkable usefulness of the parameter σM is illustrated in Fig. 3. The plot on the left shows TE for DNA complexes of several lipids, with headgroup charges ranging from +1 to +5, as a function of the cationic lipid/DOPC molar ratio [6]. The amount of DNA was kept constant for all data points. All cationic lipids exhibit a maximum in TE as a function of lipid composition: at 65 mol % for MVL2 (+2), 70 mol % for MVL3 (+3), 50 mol % for MVL5 (+5), 55 mol % for TMVL5 (+5), and 90 mol % for DOTAP (+1). This result is of note with a view to literature results which often only compare one or two ratios of cationic and neutral lipid: While the optimized TE is similar for all lipids, this TE appears at different molar ratios. Thus, testing only a few ratios is inadequate to fully assess the potential of a new lipid. The optimal molar ratios result in a TE up to three orders of magnitude larger than that of complexes which transfect poorly. The plot on the right of Fig. 3 shows the same transfection efficiency data, now plotted versus σM. Remarkably, a notable simplification takes place and all the data points merge onto a single curve. This demonstrates that σM, the membrane charge density, is a universal parameter and a predictor of transfection efficiency for lamellar (LαC) CL–DNA complexes. The resulting universal curve reveals an optimal charge density of σM* = 17.0±0.1 ×10–3 (e/Å2) [6].
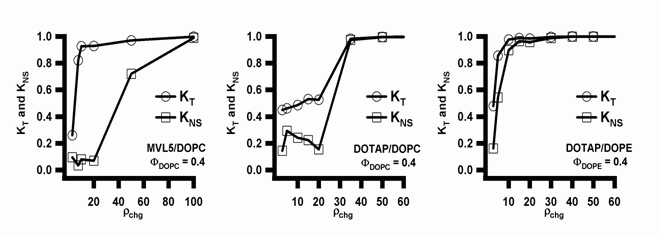
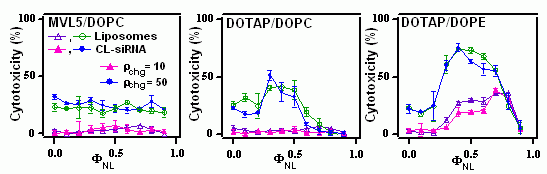
X-ray diffraction shows that DOTAP as well as the MVLs form lamellar (LαC) cationic lipid/DOPC–DNA complexes. Notably, the transfection efficiency of DOTAP/DOPE containing complexes, which exhibit the HIIC phase in the low-σM region labeled Regime I in Fig. 3, is independent of σM. This deviation from the universal curve indicates a distinctly different transfection mechanism for the inverted hexagonal phase, which has also been confirmed by other methods [4]. Considering the data for DOTAP/DOPE–DNA complexes, it may be tempting to conclude that DOPE is a generally better choice of co-lipid, which eliminates the need for optimizing σM. However, independence of TE from σM is a property of complexes in the HIIC phase, not of DOPE containing complexes. New lipids, and in particular multivalent lipids with their larger headgroups (which can even be large enough to impose the HIC structure in mixtures with DOPC [3]), may result in lamellar rather than inverted hexagonal DNA complexes when mixed with DOPE. Even with DOTAP, complexes containing larger fractions of cationic lipid exhibit the lamellar phase. Furthermore, DOPE has turned out to be unsuitable for in vivo applications where cholesterol, which promotes lamellar complexes, has gained importance. It is thus important to dispel the widespread belief that complexes containing DOPE rather than DOPC as the neutral lipid are always higher transfecting. We have repeatedly shown that this assessment is not true if lipid composition is optimized, at which point DOPC-containing (lamellar, LαC) complexes transfect as well as the best DOPE-containing (HIIC) complexes [4,6]. The mechanistic implications of the data shown in Fig. 3 and detailed mechanisms for transfection with both lamellar and inverted hexagonal CL–DNA complexes have been discussed elsewhere [4,6]. In practice, to arrive at true comparisons of lipid performance, crucial parameters such as ρchg and σM must be optimized for each lipid using a reproducible, reliable transfection assay. [1] Rädler, J. O.; Koltover, I.; Salditt, T.; Safinya, C. R.: Structure of DNA-cationic liposome complexes: DNA intercalation in multilamellar membranes in distinct interhelical packing regimes. Science 1997, 275, 810-814. Cationic Lipid–siRNA Complexes In the past few years, we have expanded our research into lipid-based NA vectors by investigating delivery systems for short interfering RNA (siRNA) [1]. Introduction The recent breakthrough discoveries of the biological process of RNA interference (RNAi) and the fact that this process, which knocks out or silences a genes by cleaving the corresponding mRNAs can be triggered with short interfering RNAs (siRNAs) 19-27 base pairs long has opened up large new fields of research. RNAi can be used for basic biological studies as well as exploited for therapeutic purposes. As with DNA, the delivery of siRNAs poses major challenges. We have recently developed an assay to concomitantly but independently measure the total silencing (KT) of a targeted gene by delivered siRNA as well as the nonspecific silencing (KNS), which results from off-target effects of the siRNA, the lipid, or the vector. Since the total silencing is the sum of specific and nonspecific silencing, this method allows us to compare the ability of siRNA vectors to effectively and specifically silence the targeted gene. An ideal vector, of course, would completely and specifically silence the targeted gene (KT=1 and KNS=0). Using this methodology, we have compared our multivalent (5+) cationic lipid MVL5 (in combination with DOPC) with vectors based on the commercially available, monovalent lipid DOTAP and either DOPC or DOPE as the neutral lipid. We have also investigated the structures of CL–siRNA complexes using synchrotron x-ray diffraction. There are distinct similarities to the structures of CL–DNA complexes, such as the existence of both a lamellar and an inverted hexagonal phase. At the same time, the different geometry of the siRNAs gives rise to important differences, which include a novel and at this point unsolved structure observed with high amounts of multivalent lipid. Multivalent Lipids are Better siRNA Vectors Fig. 1 (left to right) shows plots of the total knockdown KT and nonspecific knockdown KNS as a function of ρchg at ΦNL= 0.4 for MVL5/DOPC–siRNA, DOTAP/DOPC–siRNA, and DOTAP/DOPE–siRNA complexes, respectively. The data (which look similar for ΦNL= 0.1) show that, for the lamellar pentavalent MVL5/DOPC–siRNA complexes, the nonspecific knockdown remains nearly constant and low with KNS < 0.1 for 2.8 < ρchg< 20, while KT grows rapidly to KT ≈ 0.9 (for ρchg between 10 and 15), indicative of significant sequence-specific gene silencing (Fig. 11, left). In contrast, such a region of relatively high KT and low KNS was not observed for the lamellar phases of monovalent DOTAP/DOPC–siRNA complexes, where KT never exceeds 0.55 while KNS is low (≈ 0.2) and only increases at very high ρchg, together with KNS. (Fig. 1, middle). The DOTAP/DOPE–siRNA complexes are even worse, with substantial nonspecific knockdown KNS observed even at low ρchg ≈ 5 (Fig. 1, right). In summary, the multivalent lipid synthesized by our group, MVL5 (headgroup charge = +5) [2] was found to exhibit superior silencing efficiency over a large range in the composition and ρchg phase diagram compared to monovalent DOTAP. In addtion, MVL5 was significantly less toxic.
Comparing these findings with the ones on DNA delivery, one realizes that efficient delivery of siRNAs to cells in culture requires a molar charge ratio (ρchg, cationic lipid/nucleic acid) nearly an order of magnitude larger than what is optimal for CL–DNA complexes. This larger ρchg needed for efficient silencing results in a larger amount of cationic lipid per cell. Thus, lipid toxicity may become an important issue to consider in some composition regimes since toxicity would drive KNS up. This could provide a rationale for the observation that cationic multivalent lipids (MVLs) are better vectors compared to univalent lipids, because a smaller number of MVLs is required for a given ρchg of the complex. The Source of the Nonspecific Silencing Activity As stated above, the efficiency of several of the investigated lipid vectors for siRNA were hampered by a large nonspecific component of their silencing efficiency. To investigate this further, we compared the cytotoxicity (as the presumed cause of the nonspecific silencing) of several lipid-siRNA complexes as well as the lipid mixtures used in their preparation. The results of these investigations are summarized in Figure 2. The cytotoxicity correlates well with the nonspecific silencing (KNS), which supports our hypothesis. In addition, the experiments give information about the source of the nonspecific silencing activity. In all cases, the cytotoxicity of the complexes exacly parallels that of the liposomes, which shows that the lpid is the source of the toxicity of the vectors. With the popular (for DNA delivery) helper lipid DOPE, in particular, high toxicity is observed even at charge ratios that are not toxic when using DOPC. Similarities and Differences in the Delivery of DNA and siRNA A few crucial and unexpected differences between the delivery of DNA and siRNA with lipids are the clear superiority of multivalent lipids, the uselessness of DOPE as a helper lipid for siRNA delivery, the importance of lipid toxicity for siRNA delivery, and the large difference in the ρchg required. Several of these differences are connected as we have shown above. From the point of vector formulation, DNA delivery is a simpler task (even though siRNA only has to reach the cytoplasm to be active, not the nucleus) since lipid toxicity has not been an issue with any of the lipids produced or used in our lab. In addition, our experience suggests that all cationic lipids perform similiarly well once their formulation has been optimized. Current Work / Outlook We are currently pursuing two main avenues of work with CL-siRNA vectors. In addition to exploring the effects of multivalency in more detail (using multivalent lipids other than MVL5), we are trying to find ways to reduce the high ρchg required for siRNA delivery with cationic lipids. The similarities and differences in the structures of siRNA and DNA complexes are another current field of study. [1] Bouxsein, N. F.; McAllister, C. S.; Ewert, K. K.; Samuel, C. E.; Safinya, C. R.: Structure and gene silencing activities of monovalent and pentavalent cationic lipid vectors complexed with siRNA. Biochemistry 2007, 46, 4785-4792. Dendrimer–DNA Complexes The second major branch of nonviral gene delivery methods employs complexes of DNA and positively charged macromolecules. These comprise not only linear or hyperbranched polymers, but also cationic dendrimers. We have studied DNA vectors based on poly(propylen imine) (PPI) dendrimers using x-ray diffraction, microscopy, and biological efficiency assays. In this system, electrostatic and entropic forces cause the DNA and the positively charged molecules to self-assemble into compact structures that sometimes display long-range order. These complexes can have a (weak) positive surface charge, leading to electrostatic attraction to negatively charged components of cellular membranes, thereby increasing the likelihood of endocytosis and subsequent gene expression. In addition, dendrimers are monodisperse cationic units of variable size that are useful as a simple system to study the interactions between DNA and polyvalent molecules and even histones. We have found that both hexagonal and rectangular phases of PPI dendrimer-DNA complexes are formed, depending on the size (generation) of the dendrimer and the dendrimer/DNA charge ratio. In collaboration with theoretical physicist R. Bruinsma, we were able to explain these unexpected findings using a simple model based on competing long-range electrostatic interactions and short-range entropic adhesion by counterion release.
For more information: Evans, H. M.; Ahmad, A.; Ewert, K.; Pfohl, T.; Martin-Herranz, A.; Bruinsma, R. F.; Safinya, C. R.: Structural polymorphism of DNA-dendrimer complexes. Phys. Rev. Lett. 2003, 91, 075501.
|